Trimming and Filtering
Overview
Teaching: 30 min
Exercises: 25 minQuestions
How can I get rid of sequence data that does not meet my quality standards?
Objectives
Clean FASTQ reads using Trimmomatic.
Select and set multiple options for command-line bioinformatic tools.
Write
forloops with two variables.
Cleaning reads
In the previous episode, we took a high-level look at the quality of each of our samples using FastQC. We visualized per-base quality graphs showing the distribution of read quality at each base across all reads in a sample and extracted information about which samples fail which quality checks. Some of our samples failed quite a few quality metrics used by FastQC. This does not mean, though, that our samples should be thrown out! It is very common to have some quality metrics fail, and this may or may not be a problem for your downstream application. For our variant calling workflow, we will be removing some of the low quality sequences to reduce our false positive rate due to sequencing error.
We will use a program called Trimmomatic to filter poor quality reads and trim poor quality bases from our samples.
Trimmomatic options
Trimmomatic has a variety of options to trim your reads. If we run the following command, we can see some of our options.
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
trimmomatic
Which will give you the following output:
Usage:
PE [-version] [-threads <threads>] [-phred33|-phred64] [-trimlog <trimLogFile>] [-summary <statsSummaryFile>] [-quiet] [-validatePairs] [-basein <inputBase> | <inputFile1> <inputFile2>] [-baseout <outputBase> | <outputFile1P> <outputFile1U> <outputFile2P> <outputFile2U>] <trimmer1>...
or:
SE [-version] [-threads <threads>] [-phred33|-phred64] [-trimlog <trimLogFile>] [-summary <statsSummaryFile>] [-quiet] <inputFile> <outputFile> <trimmer1>...
or:
-version
This output shows us that we must first specify whether we have paired end (PE) or single end (SE) reads.
Next, we specify what flag we would like to run. For example, you can specify threads to indicate the number of
processors on your computer that you want Trimmomatic to use. In most cases using multiple threads (processors) can help to run the trimming faster. These flags are not necessary, but they can give you more control over the command. The flags are followed by positional arguments, meaning the order in which you specify them is important.
In paired end mode, Trimmomatic expects the two input files, and then the names of the output files. These files are described below. While, in single end mode, Trimmomatic will expect 1 file as input, after which you can enter the optional settings and lastly the name of the output file.
| option | meaning |
|---|---|
| <inputFile1> | Input reads to be trimmed. Typically the file name will contain an _1 or _R1 in the name. |
| <inputFile2> | Input reads to be trimmed. Typically the file name will contain an _2 or _R2 in the name. |
| <outputFile1P> | Output file that contains surviving pairs from the _1 file. |
| <outputFile1U> | Output file that contains orphaned reads from the _1 file. |
| <outputFile2P> | Output file that contains surviving pairs from the _2 file. |
| <outputFile2U> | Output file that contains orphaned reads from the _2 file. |
Two important parameters that we’ll use to keep our data organized in our directory structure:
| option | meaning |
|---|---|
| -basein <inputBase> | This will automatically prepend our input file names with a value. Ours is -basein ../01_input/untrimmed_fastq/ |
| -baseout <outputBase> | This will automatically prepend our output file names with a value. Ours is -baseout ../03_output |
The last thing trimmomatic expects to see is the trimming parameters:
| step | meaning |
|---|---|
ILLUMINACLIP |
Perform adapter removal. |
SLIDINGWINDOW |
Perform sliding window trimming, cutting once the average quality within the window falls below a threshold. |
LEADING |
Cut bases off the start of a read, if below a threshold quality. |
TRAILING |
Cut bases off the end of a read, if below a threshold quality. |
CROP |
Cut the read to a specified length. |
HEADCROP |
Cut the specified number of bases from the start of the read. |
MINLEN |
Drop an entire read if it is below a specified length. |
TOPHRED33 |
Convert quality scores to Phred-33. |
TOPHRED64 |
Convert quality scores to Phred-64. |
We will use only a few of these options and trimming steps in our analysis. It is important to understand the steps you are using to clean your data. For more information about the Trimmomatic arguments and options, see the Trimmomatic manual.
However, a complete command for Trimmomatic will look something like the command below. This command is an example and will not work, as we do not have the files it refers to:
# only an example command
trimmomatic PE -threads 4 SRR_1056_1.fastq SRR_1056_2.fastq \
SRR_1056_1.trimmed.fastq SRR_1056_1un.trimmed.fastq \
SRR_1056_2.trimmed.fastq SRR_1056_2un.trimmed.fastq \
ILLUMINACLIP:SRR_adapters.fa SLIDINGWINDOW:4:20
In this example, we have told Trimmomatic:
| code | meaning |
|---|---|
PE |
that it will be taking a paired end file as input |
-threads 4 |
to use four computing threads to run (this will speed up our run) |
SRR_1056_1.fastq |
the first input file name |
SRR_1056_2.fastq |
the second input file name |
SRR_1056_1.trimmed.fastq |
the output file for surviving pairs from the _1 file |
SRR_1056_1un.trimmed.fastq |
the output file for orphaned reads from the _1 file |
SRR_1056_2.trimmed.fastq |
the output file for surviving pairs from the _2 file |
SRR_1056_2un.trimmed.fastq |
the output file for orphaned reads from the _2 file |
ILLUMINACLIP:SRR_adapters.fa |
to clip the Illumina adapters from the input file using the adapter sequences listed in SRR_adapters.fa |
SLIDINGWINDOW:4:20 |
to use a sliding window of size 4 that will remove bases if their phred score is below 20 |
Running Trimmomatic
Now we will run Trimmomatic on our data. But first, explore the directory pointed to by $CONDA_PREFIX to get data that was installed with trimmomatic.
Exploring your conda environment/installation
Understanding more about conda
We know that we’ve created conda environments and added software to them, but we don’t see what conda is doing.
One way to look is to explore the directories where conda does its work.
This section doesn’t require being in a specific directory, because all commands will deal with absolute paths.
Absolute paths fully describe the
location of a file or directory, and are valid regardless of your current working directory. You can identify an absolute path because it always starts with a /. We have mainly dealt with relative paths so far, which can contain slashes, but never at the very beginning.
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
echo $CONDA_PREFIX
David’s output
/projects/.colostate.edu/dcking/software/anaconda/envs/qc-trim
This is a subdirectory of my /projects directory that is just for the environment qc-trim. All programs data I installed into this environment will be in here. Let’s explore it. Yours may differ for some packages
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
ls $CONDA_PREFIX
David’s output
bin conda-meta etc include legal libexec opt share var x86_64-conda-linux-gnu
compiler_compat conf fonts jmods lib man release ssl x86_64-conda_cos7-linux-gnu
Installed programs are in bin, whereas installed data is typical in share.
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
ls $CONDA_PREFIX/share
My share directory:
aclocal dbus-1 fontconfig glib-2.0 info locale tabset trimmomatic xml
bash-completion doc gettext icu licenses man terminfo trimmomatic-0.39-2 zoneinfo
There are two trimmomatic directories, but trimmomatic is just a link (alias) to trimmomatic-0.39-2.
# don't need to be in a specific directory
total 568
drwxr-xr-x. 2 dcking@colostate.edu erinnishgrp@colostate.edu 149 Apr 16 20:01 aclocal
drwxr-xr-x. 3 dcking@colostate.edu erinnishgrp@colostate.edu 29 Apr 16 19:58 bash-completion
drwxr-xr-x. 2 dcking@colostate.edu erinnishgrp@colostate.edu 59 Apr 16 19:58 dbus-1
drwxr-xr-x. 6 dcking@colostate.edu erinnishgrp@colostate.edu 91 Apr 16 19:58 doc
drwxr-xr-x. 3 dcking@colostate.edu erinnishgrp@colostate.edu 28 Apr 16 20:01 fontconfig
drwxr-xr-x. 3 dcking@colostate.edu erinnishgrp@colostate.edu 21 Apr 16 19:58 gettext
drwxr-xr-x. 6 dcking@colostate.edu erinnishgrp@colostate.edu 97 Apr 16 19:58 glib-2.0
drwxr-xr-x. 3 dcking@colostate.edu erinnishgrp@colostate.edu 22 Apr 16 20:01 icu
drwxr-xr-x. 2 dcking@colostate.edu erinnishgrp@colostate.edu 213 Apr 16 20:01 info
drwxr-xr-x. 4 dcking@colostate.edu erinnishgrp@colostate.edu 53 Apr 16 19:57 licenses
drwxr-xr-x. 27 dcking@colostate.edu erinnishgrp@colostate.edu 509 Apr 16 20:01 locale
drwxr-xr-x. 8 dcking@colostate.edu erinnishgrp@colostate.edu 128 Apr 16 20:01 man
drwxr-xr-x. 2 dcking@colostate.edu erinnishgrp@colostate.edu 91 Apr 16 19:57 tabset
drwxr-xr-x. 44 dcking@colostate.edu erinnishgrp@colostate.edu 798 Apr 16 19:57 terminfo
lrwxrwxrwx. 1 dcking@colostate.edu erinnishgrp@colostate.edu 18 Apr 16 19:58 trimmomatic -> trimmomatic-0.39-2
drwxr-xr-x. 3 dcking@colostate.edu erinnishgrp@colostate.edu 224 Apr 16 19:58 trimmomatic-0.39-2
drwxr-xr-x. 4 dcking@colostate.edu erinnishgrp@colostate.edu 52 Apr 16 20:01 xml
drwxr-xr-x. 19 dcking@colostate.edu erinnishgrp@colostate.edu 1590 Apr 16 19:57 zoneinfo
Explore trimmomatic
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
ls $CONDA_PREFIX/share/trimmomatic
adapters build_env_setup.sh conda_build.sh LICENSE metadata_conda_debug.yaml trimmomatic trimmomatic.jar
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
ls $CONDA_PREFIX/share/trimmomatic/adapters
NexteraPE-PE.fa TruSeq2-PE.fa TruSeq2-SE.fa TruSeq3-PE-2.fa TruSeq3-PE.fa TruSeq3-SE.fa
WE FOUND IT The adapter sequences are just short fasta format sequences
# don't need to be in a particular directory, but need env:
# conda activate qc-trim
cat $CONDA_PREFIX/share/trimmomatic/adapters/NexteraPE-PE.fa
>PrefixNX/1
AGATGTGTATAAGAGACAG
>PrefixNX/2
AGATGTGTATAAGAGACAG
>Trans1
TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG
>Trans1_rc
CTGTCTCTTATACACATCTGACGCTGCCGACGA
>Trans2
GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG
>Trans2_rc
CTGTCTCTTATACACATCTCCGAGCCCACGAGAC
We are going to run Trimmomatic on one of our paired-end samples. While using FastQC we saw that Nextera adapters were present in our samples. The adapter sequences came with the installation of trimmomatic, so we will first copy these sequences into our current directory.
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC
cd ../01_input/untrimmed_fastq
cp $CONDA_PREFIX/share/trimmomatic/adapters/NexteraPE-PE.fa .
ls
NexteraPE-PE.fa SRR2584863_2.fastq SRR2584866_1.fastq.gz SRR2589044_1.fastq SRR2589044_2.fastq.gz
SRR2584863_1.fastq SRR2584863_2.fastq.gz SRR2584866_2.fastq SRR2589044_1.fastq.gz
SRR2584863_1.fastq.gz SRR2584866_1.fastq SRR2584866_2.fastq.gz SRR2589044_2.fastq
New command template (this is not the actual command)
trimmomatic PE -threads 4 SRR_1056_1.fastq SRR_1056_2.fastq \
SRR_1056_1.trimmed.fastq SRR_1056_1un.trimmed.fastq \
SRR_1056_2.trimmed.fastq SRR_1056_2un.trimmed.fastq \
ILLUMINACLIP:SRR_adapters.fa SLIDINGWINDOW:4:20
- We will: use a sliding window of size 4 that will remove bases if their phred score is below 20 (like in our example above).
- … also discard any reads that do not have at least 25 bases remaining after this trimming step.
- Three additional pieces of code are also added to the end of the ILLUMINACLIP step.
- These three additional numbers (2:40:15) tell Trimmomatic how to handle sequence matches to the Nextera adapters.
- A detailed explanation of how they work is advanced for this particular lesson.
- For now we will use these numbers as a default and recognize they are needed to for Trimmomatic to run properly.
This command will take a few minutes to run. (faster on Alpine)
Script version 1
Make a script called trimmomatic.sbatch.
#!/usr/bin/env bash
#SBATCH --nodes=1
#SBATCH --ntasks=1
#SBATCH --time=0:10:00
#SBATCH --partition=amilan
#SBATCH --qos=normal
#SBATCH --job-name=trimmomatic
source /curc/sw/anaconda3/latest
conda activate qc-trim
IN=../01_input/untrimmed_fastq
OUT=../03_output/trimmed_reads
adapters_fa=$IN/NexteraPE-PE.fa
mkdir -p $OUT
trimmomatic PE \
$IN/SRR2589044_1.fastq.gz $IN/SRR2589044_2.fastq.gz \
$OUT/SRR2589044_1.trim.fastq.gz $OUT/SRR2589044_1un.trim.fastq.gz \
$OUT/SRR2589044_2.trim.fastq.gz $OUT/SRR2589044_2un.trim.fastq.gz \
SLIDINGWINDOW:4:20 \
MINLEN:25 \
ILLUMINACLIP:$adapters_fa:2:40:15
Run the script:
sbatch trimmomatic.sbatch
Submitted batch job 1141244
Script: 2nd approach uses “accession” but only does one sample
Using variables and string functions to generalize a script
You can generalize your script by replacing explicit values in your code with that of a variable. You set the variable before using it, so, when you want to change your script to work on a different value, that value only needs to be changed in one place.
filename=SRR2589044_1.fastq.gz # want to remove "_1.fastq.gz" accession=$(basename $filename _1.fastq.gz} echo "processing accession $accession" # prints: processing accession SRR2589044 # replace all previous occurrences of SRR2589044 with ${accession} trimmomatic PE \ $IN/${accession}_1.fastq.gz $IN/${accession}_2.fastq.gz \ $OUT/${accession}_1.trim.fastq.gz $OUT/${accession}_1un.trim.fastq.gz \ $OUT/${accession}_2.trim.fastq.gz $OUT/${accession}_2un.trim.fastq.gz \ SLIDINGWINDOW:4:20 \ MINLEN:25 \ ILLUMINACLIP:$adapters_fa:2:40:15You have to do
${accession}to separate the variable name from the trailing underscores (i.e.${accession}_)
Run the script:
sbatch trimmomatic.sbatch
Submitted batch job 1141303
The output from trimmomatic is will be in a log file (slurm-1141303.out) like this:
TrimmomaticPE: Started with arguments:
SRR2589044_1.fastq.gz SRR2589044_2.fastq.gz SRR2589044_1.trim.fastq.gz SRR2589044_1un.trim.fastq.gz SRR2589044_2.trim.fastq.gz SRR2589044_2un.trim.fastq.gz SLIDINGWINDOW:4:20 MINLEN:25 ILLUMINACLIP:NexteraPE-PE.fa:2:40:15
Multiple cores found: Using 2 threads
Using PrefixPair: 'AGATGTGTATAAGAGACAG' and 'AGATGTGTATAAGAGACAG'
Using Long Clipping Sequence: 'GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG'
Using Long Clipping Sequence: 'TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG'
Using Long Clipping Sequence: 'CTGTCTCTTATACACATCTCCGAGCCCACGAGAC'
Using Long Clipping Sequence: 'CTGTCTCTTATACACATCTGACGCTGCCGACGA'
ILLUMINACLIP: Using 1 prefix pairs, 4 forward/reverse sequences, 0 forward only sequences, 0 reverse only sequences
Quality encoding detected as phred33
Input Read Pairs: 1107090 Both Surviving: 885220 (79.96%) Forward Only Surviving: 216472 (19.55%) Reverse Only Surviving: 2850 (0.26%) Dropped: 2548 (0.23%)
TrimmomaticPE: Completed successfully
Exercise
Use the output from your Trimmomatic command to answer the following questions.
1) What percent of reads did we discard from our sample? 2) What percent of reads did we keep both pairs?
Solution
1) 0.23% 2) 79.96%
You may have noticed that Trimmomatic automatically detected the quality encoding of our sample. It is always a good idea to double-check this or to enter the quality encoding manually.
We can confirm that we have our output files:
ls ../03_output/trimmed_reads/SRR2589044* -l -h
-rw-r--r--. 1 dcking@colostate.edu dckingpgrp@colostate.edu 94M Apr 18 21:28 ../03_output/trimmed_reads/SRR2589044_1.trim.fastq.gz
-rw-r--r--. 1 dcking@colostate.edu dckingpgrp@colostate.edu 18M Apr 18 21:28 ../03_output/trimmed_reads/SRR2589044_1un.trim.fastq.gz
-rw-r--r--. 1 dcking@colostate.edu dckingpgrp@colostate.edu 91M Apr 18 21:28 ../03_output/trimmed_reads/SRR2589044_2.trim.fastq.gz
-rw-r--r--. 1 dcking@colostate.edu dckingpgrp@colostate.edu 271K Apr 18 21:28 ../03_output/trimmed_reads/SRR2589044_2un.trim.fastq.gz
We have just successfully run Trimmomatic on one of our FASTQ files!
However, there is some bad news. Trimmomatic can only operate on
one sample at a time and we have more than one sample. The good news
is that we can use a for loop to iterate through our sample files
quickly!
Script version 3: add a loop to handle all samples in one job
#!/usr/bin/env bash
#SBATCH --nodes=1
#SBATCH --ntasks=1
#SBATCH --time=0:10:00
#SBATCH --partition=amilan
#SBATCH --qos=normal
#SBATCH --job-name=trimmomatic
source /curc/sw/anaconda3/latest
conda activate qc-trim
adapters_fa=../01_input/untrimmed_fastq/NexteraPE-PE.fa
IN=../01_input/untrimmed_fastq
OUT=../03_output/trimmed_reads
mkdir -p $OUT
for infile in $IN/*_1.fastq.gz
do
accession=$(basename $infile _1.fastq.gz)
echo "processing accession $accession"
trimmomatic PE \
$IN/${accession}_1.fastq.gz $IN/${accession}_2.fastq.gz \
$OUT/${accession}_1.trim.fastq.gz $OUT/${accession}_1un.trim.fastq.gz \
$OUT/${accession}_2.trim.fastq.gz $OUT/${accession}_2un.trim.fastq.gz \
SLIDINGWINDOW:4:20 \
MINLEN:25 \
ILLUMINACLIP:$adapters_fa:2:40:15
done
Go ahead and run the for loop. It should take a few minutes for
Trimmomatic to run for each of our six input files. Once it is done
running, take a look at your directory contents. You will notice that even though we ran Trimmomatic on file SRR2589044 before running the for loop, there is only one set of files for it. Because we matched the ending _1.fastq.gz, we re-ran Trimmomatic on this file, overwriting our first results. That is ok, but it is good to be aware that it happened.
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
cd ../03_output/trimmed_reads
ls
SRR2584863_1.trim.fastq.gz SRR2584863_2un.trim.fastq.gz SRR2584866_2.trim.fastq.gz SRR2589044_1un.trim.fastq.gz
SRR2584863_1un.trim.fastq.gz SRR2584866_1.trim.fastq.gz SRR2584866_2un.trim.fastq.gz SRR2589044_2.trim.fastq.gz
SRR2584863_2.trim.fastq.gz SRR2584866_1un.trim.fastq.gz SRR2589044_1.trim.fastq.gz SRR2589044_2un.trim.fastq.gz
make use of multiple threads
Notice the addition of -threads, passing it the value of $SLURM_NTASKS.
You can change –ntasks=2. Try 4, or 6.
Script version 3.5: loop approach but with -threads
#!/usr/bin/env bash
#SBATCH --nodes=1
#SBATCH --ntasks=2
#SBATCH --time=0:10:00
#SBATCH --partition=amilan
#SBATCH --qos=normal
#SBATCH --job-name=trimmomatic
source /curc/sw/anaconda3/latest
conda activate qc-trim
adapters_fa=../01_input/untrimmed_fastq/NexteraPE-PE.fa
IN=../01_input/untrimmed_fastq
OUT=../03_output/trimmed_reads
mkdir -p $OUT
for infile in $IN/*_1.fastq.gz
do
accession=$(basename $infile _1.fastq.gz)
echo "processing accession $accession"
trimmomatic PE \
-threads $SLURM_NTASKS\
$IN/${accession}_1.fastq.gz $IN/${accession}_2.fastq.gz \
$OUT/${accession}_1.trim.fastq.gz $OUT/${accession}_1un.trim.fastq.gz \
$OUT/${accession}_2.trim.fastq.gz $OUT/${accession}_2un.trim.fastq.gz \
SLIDINGWINDOW:4:20 \
MINLEN:25 \
ILLUMINACLIP:$adapters_fa:2:40:15
done
optional - array version
Script version 4: one job per sample in an array
#!/usr/bin/env bash
#SBATCH --nodes=1
#SBATCH --ntasks=4
#SBATCH --time=0:02:00
#SBATCH --partition=amilan
#SBATCH --qos=normal
#SBATCH --job-name=trimmomatic
# to submit this script, you do sbatch --array=0-2 trimmomatic.sbatch
source /curc/sw/anaconda3/latest
conda activate qc-trim
adapters_fa=../01_input/untrimmed_fastq/NexteraPE-PE.fa
IN=../01_input/untrimmed_fastq
OUT=../03_output/trimmed_reads
mkdir -p $OUT
infiles=( $IN/*_1.fastq.gz )
infile=${infiles[$SLURM_ARRAY_TASK_ID]}
accession=$(basename $infile _1.fastq.gz)
echo "processing accession $accession"
trimmomatic PE \
-threads $SLURM_NTASKS\
$IN/${accession}_1.fastq.gz $IN/${accession}_2.fastq.gz \
$OUT/${accession}_1.trim.fastq.gz $OUT/${accession}_1un.trim.fastq.gz \
$OUT/${accession}_2.trim.fastq.gz $OUT/${accession}_2un.trim.fastq.gz \
SLIDINGWINDOW:4:20 \
MINLEN:25 \
ILLUMINACLIP:$adapters_fa:2:40:15
sbatch --array=0-2 trimmomatic.sbatch
Submitted batch job 1141362
Checking job status:
sq
Array job pending:
Tue Apr 18 22:01:59 2023
JOBID PARTITION NAME USER ST TIME NODES NODELIST(REASON)
1141362_[0-2] amilan trimmoma dcking@c PD 0:00 1 (Priority)
Array job running:
Tue Apr 18 22:02:19 2023
JOBID PARTITION NAME USER ST TIME NODES NODELIST(REASON)
1141362_0 amilan trimmoma dcking@c R 0:09 1 c3cpu-c11-u1-3
1141362_1 amilan trimmoma dcking@c R 0:09 1 c3cpu-c11-u1-3
1141362_2 amilan trimmoma dcking@c R 0:09 1 c3cpu-c11-u1-3
Comparison between single job, 4-6 threads, and array job, 4 threads:
sa
1141352 trimmomat+ 4 COMPLETED 0:0 00:02:07 00:10:00 2023-04-18T21:51:20 2023-04-18T21:51:43 2023-04-18T21:53:50
1141360 trimmomat+ 6 COMPLETED 0:0 00:01:58 00:10:00 2023-04-18T21:54:19 2023-04-18T21:55:17 2023-04-18T21:57:15
1141362_0 trimmomat+ 4 COMPLETED 0:0 00:00:44 00:02:00 2023-04-18T22:01:15 2023-04-18T22:02:10 2023-04-18T22:02:54
1141362_1 trimmomat+ 4 COMPLETED 0:0 00:01:00 00:02:00 2023-04-18T22:01:15 2023-04-18T22:02:10 2023-04-18T22:03:10
1141362_2 trimmomat+ 4 COMPLETED 0:0 00:00:29 00:02:00 2023-04-18T22:01:15 2023-04-18T22:02:10 2023-04-18T22:02:39
Maintain data integrity with checksums
md5sum * > checksum.md5
cat checksum.md5
ada4ddd4e2f02671786491a793d3b380 SRR2584863_1.trim.fastq.gz
50045bcaab602667a95f53870002e68d SRR2584863_1un.trim.fastq.gz
7c22ca7cd60030160f6425d9e6bc75ea SRR2584863_2.trim.fastq.gz
c6314804acac3a013f951d6fb6fe2d52 SRR2584863_2un.trim.fastq.gz
9bf83b31d7882f637f57d0ecac2e288f SRR2584866_1.trim.fastq.gz
57ed6d9cf50697693127c76d41ec074e SRR2584866_1un.trim.fastq.gz
e6cd3bdc17af0b5779fb3f40ff8e280e SRR2584866_2.trim.fastq.gz
84ad1a8cd661253c25a653c3cb5b364b SRR2584866_2un.trim.fastq.gz
e029c09c402be9e8d9703ebd74c6b5b0 SRR2589044_1.trim.fastq.gz
c5d43e3d4ae406b34019cf290c795f9e SRR2589044_1un.trim.fastq.gz
7d29399f60affb38e3439d3132857363 SRR2589044_2.trim.fastq.gz
375e9041a385a39571ac0672e4ed764c SRR2589044_2un.trim.fastq.gz
md5sum -c checksum.md5
SRR2584863_1.trim.fastq.gz: OK
SRR2584863_1un.trim.fastq.gz: OK
SRR2584863_2.trim.fastq.gz: OK
SRR2584863_2un.trim.fastq.gz: OK
SRR2584866_1.trim.fastq.gz: OK
SRR2584866_1un.trim.fastq.gz: OK
SRR2584866_2.trim.fastq.gz: OK
SRR2584866_2un.trim.fastq.gz: OK
SRR2589044_1.trim.fastq.gz: OK
SRR2589044_1un.trim.fastq.gz: OK
SRR2589044_2.trim.fastq.gz: OK
SRR2589044_2un.trim.fastq.gz: OK
Debugging and troubleshooting on the command line
Install tree.
You can’t install into base: use one of your environments.
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
conda activate qc-trim
conda install tree
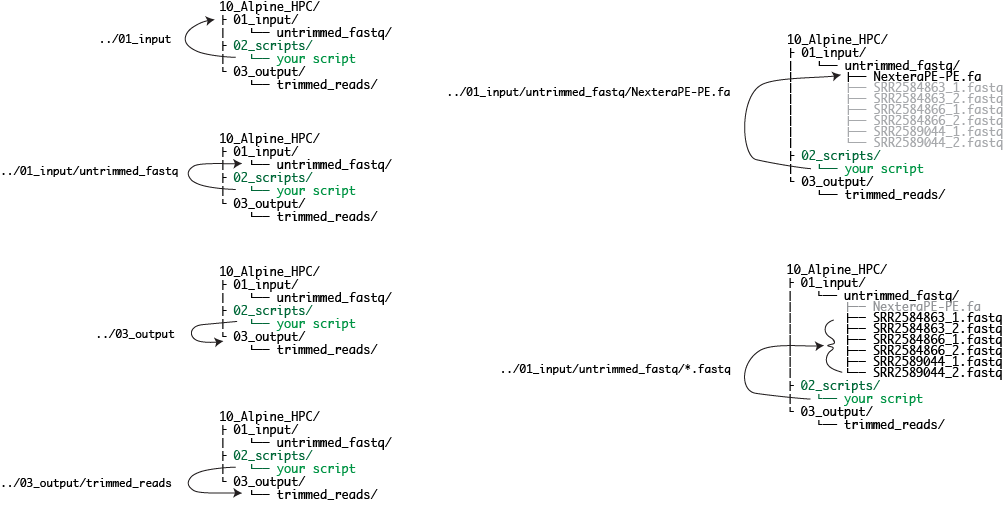
verify paths using ls
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
IN=../01_input/untrimmed_fastq
ls $IN
NexteraPE-PE.fa SRR2584863_1.fastq SRR2584863_2.fastq SRR2584866_1.fastq SRR2584866_2.fastq SRR2589044_1.fastq SRR2589044_2.fastq
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
adapters_fa=$IN/NexteraPE-PE.fa
ls $adapters_fa
../01_input/untrimmed_fastq/NexteraPE-PE.fa
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
ls $IN/*.fastq
../01_input/untrimmed_fastq/SRR2584863_1.fastq ../01_input/untrimmed_fastq/SRR2584866_1.fastq ../01_input/untrimmed_fastq/SRR2589044_1.fastq
../01_input/untrimmed_fastq/SRR2584863_2.fastq ../01_input/untrimmed_fastq/SRR2584866_2.fastq ../01_input/untrimmed_fastq/SRR2589044_2.fastq
try basename on paths and file extensions
Isolate the file part of a path with basename path.
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
basename $IN/SRR2589044_1.fastq
SRR2589044_1.fastq
Remove a suffix or extension of a filename with basename filename.ext .ext
basename SRR2589044_1.fastq _1.fastq
SRR2589044
Remove both the leading directories AND the file extension at the same time.
# cd /projects/$USER/CM580A3-Intro-to-qCMB-2023/10_Alpine_HPC/02_scripts
basename $IN/SRR2589044_1.fastq _1.fastq
SRR2589044
test more statements from the script
The output from the string functions must be stored if we plan to modify them in order to form new filenames.
savedoutput=$( command )
filename=SRR2589044_1.fastq
accession=$(basename $filename _1.fastq)
echo $filename
echo $accession
SRR2589044_1.fastq
SRR2589044
ls $IN/${accession}_2.fastq
../01_input/untrimmed_fastq/SRR2589044_2.fastq
Notice that we referred to _2.fastq, but we got the accession ID from the corresponding _1.fastq.
When there’s an error (accession mispelled/typo):
ls $IN/${acession}_2.fastq
ls: cannot access ../01_input/untrimmed_fastq/_2.fastq: No such file or directory
Interactive compute session with ainteractive
(base) [dcking@colostate.edu@login12 02_scripts]$ ainteractive
ainteractive: submitting job... salloc --nodes=1 --ntasks=1 --partition=ainteractive --time=1:00:00 --bell srun --pty /bin/bash
salloc: Granted job allocation 1158992
salloc: Waiting for resource configuration
salloc: Nodes c3cpu-a7-u34-2 are ready for job
(base) [dcking@colostate.edu@c3cpu-a7-u34-2 02_scripts]$
You can now test your script using bash scriptname instead of sbatch scriptname and get your output to the screen.
Benefits:
- waiting in the queue with full resource requests
- waiting for your job to complete
- output to your screen instead of looking for the slurm-123456.out file
- faster edit/test pace
Once you have your script running properly, finding all of the files, etc., then you can exit the interactive session.
(base) [dcking@colostate.edu@c3cpu-a7-u34-2 02_scripts]$ exit
exit
salloc: Relinquishing job allocation 1158992
(base) [dcking@colostate.edu@login12 02_scripts]$
Bash settings and flags for debugging
These commands can go after the #SBATCH headers
set -e : quit on first error
set -u : quit if an unset variable is accessed - for example ${acession} when accession is variable you set
From the command line
bash -n scriptname: don’t run the script, but check for syntax errors
If you are in ainteractive and are testing with bash scriptname instead of sbatch scriptname:
bash -v scriptname: print out each line as it executes.
bash -x scriptname: like -v, but expands commands. (I find this output difficult to read).
General tips
Use lots of echo statements in the script to check variables
echo "accession is set to $accession"
echo "IN is set to $IN"
echo "listing IN ($IN):"
ls $IN
listing IN (../01_input/untrimmed_fastq):
NexteraPE-PE.fa SRR2584863_1.fastq SRR2584863_2.fastq SRR2584866_1.fastq SRR2584866_2.fastq SRR2589044_1.fastq SRR2589044_2.fastq
Key Points
The options you set for the command-line tools you use are important!
Data cleaning is an essential step in a genomics workflow.